Acyclovir (Compound)
Analyze
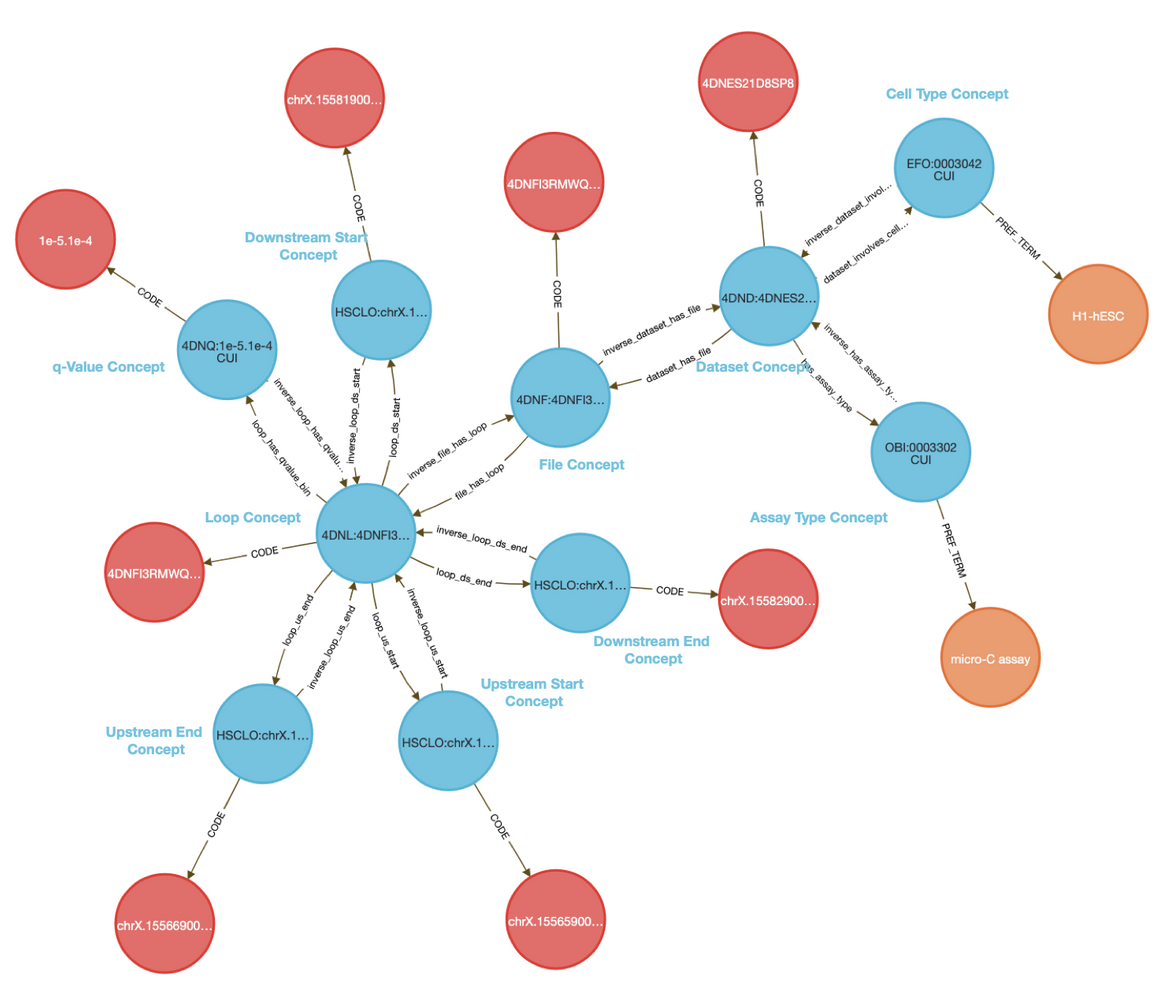
The CFDE Data Distillery Knowledge Graph contains entities and relationships across the CFDE. View Acyclovir's neighborhood in the knowledge graph.
The Gene and Drug Landing Page Aggregator (GDLPA) finds links to primary and secondary source information from CFDE and other resources. Discover landing pages for Acyclovir.
The Playbook Workflow Builder helps you interactively construct workflows leveraging CFDE APIs without code. Start a new workflow with Acyclovir.
Results found
Linked to
| Label | Description |
|
|---|---|---|---|
A Substance | |||
Metadatata for acyclovir | |||
DrugPage Collection: (DrugCentralID:00085; PubChem_CID:135398513, FILE=drugcentral_drug_00085.json) | |||
A Knowledge Graph Assertion from LINCS | |||
A Knowledge Graph Assertion from LINCS | |||
A Knowledge Graph Assertion from LINCS | |||
A Knowledge Graph Assertion from LINCS | |||
A Knowledge Graph Assertion from LINCS | |||
A Knowledge Graph Assertion from LINCS | |||
A Knowledge Graph Assertion from LINCS |
Metadatata for acyclovir
DrugPage Collection: (DrugCentralID:00085; PubChem_CID:135398513, FILE=drugcentral_drug_00085.json)
A Knowledge Graph Assertion from LINCS
A Knowledge Graph Assertion from LINCS
A Knowledge Graph Assertion from LINCS
A Knowledge Graph Assertion from LINCS
A Knowledge Graph Assertion from LINCS
A Knowledge Graph Assertion from LINCS
A Knowledge Graph Assertion from LINCS