Cyclic gmp (Compound)
Synonyms:40732-48-7, Monosodium-GMP, Guanosine 3',5'-cyclic monophosphate sodium salt, cyclic gmp, UNII-6WYT1TNR45, Sodium guanosine cyclic monophosphate, Guanosine Monophosphate Monosodium Salt, Cyclic gmp sodium salt, 6WYT1TNR45, GUANOSINE3:5-CYCLICMONOPHOSPHATESODIUMSALT, MFCD00038424, sodium;9-[(4aR,6R,7R,7aS)-7-hydroxy-2-oxido-2-oxo-4a,6,7,7a-tetrahydro-4H-furo[3,2-d][1,3,2]dioxaphosphinin-6-yl]-2-amino-3H-purin-6-one, Guanosine-3':5'-cyclic monophosphate monosodium salt, cGMP sodium salt, EINECS 255-056-0, Cyclic 3',5'-Guanosine Monophosphate Monosodium Salt, 3',5'-cGMP-Na, C10H11N5O7P.Na, DTXSID30860018, Guanosine, cyclic 3',5'-(hydrogen phosphate), monosodium salt, 3',5'-Cyclic GMP monosodium salt, 7032AH, AKOS037646872, AS-71285, Guanosine 3',5'-cyclophosphate monosodium salt, Guanosine 3:5-cyclic monophosphate sodium salt, guanosine 3',5'-cyclic-monophosphate sodium salt, GUANOSINE 3':5'-CYCLIC MONOPHOSPHATE SOD, Q27265640, Guanosine 3',5'-cyclic monophosphate sodium salt, >=99.0% (HPLC), Guanosine 3',5'-cyclic monophosphate sodium salt, >=99% (HPLC), powder, Guanosine 3',5'-cyclic Monophosphate, Sodium Salt - CAS 40732-48-7, sodium (4aR,6R,7R,7aS)-6-(2-amino-6-oxo-6,9-dihydro-1H-purin-9-yl)-7-hydroxy-2-oxo-hexahydro-2??-furo[3,2-d][1,3,2]dioxaphosphinin-2-olate
Pubchem:PUBCHEM:24316
Id:135445730
Description:nan
Analyze
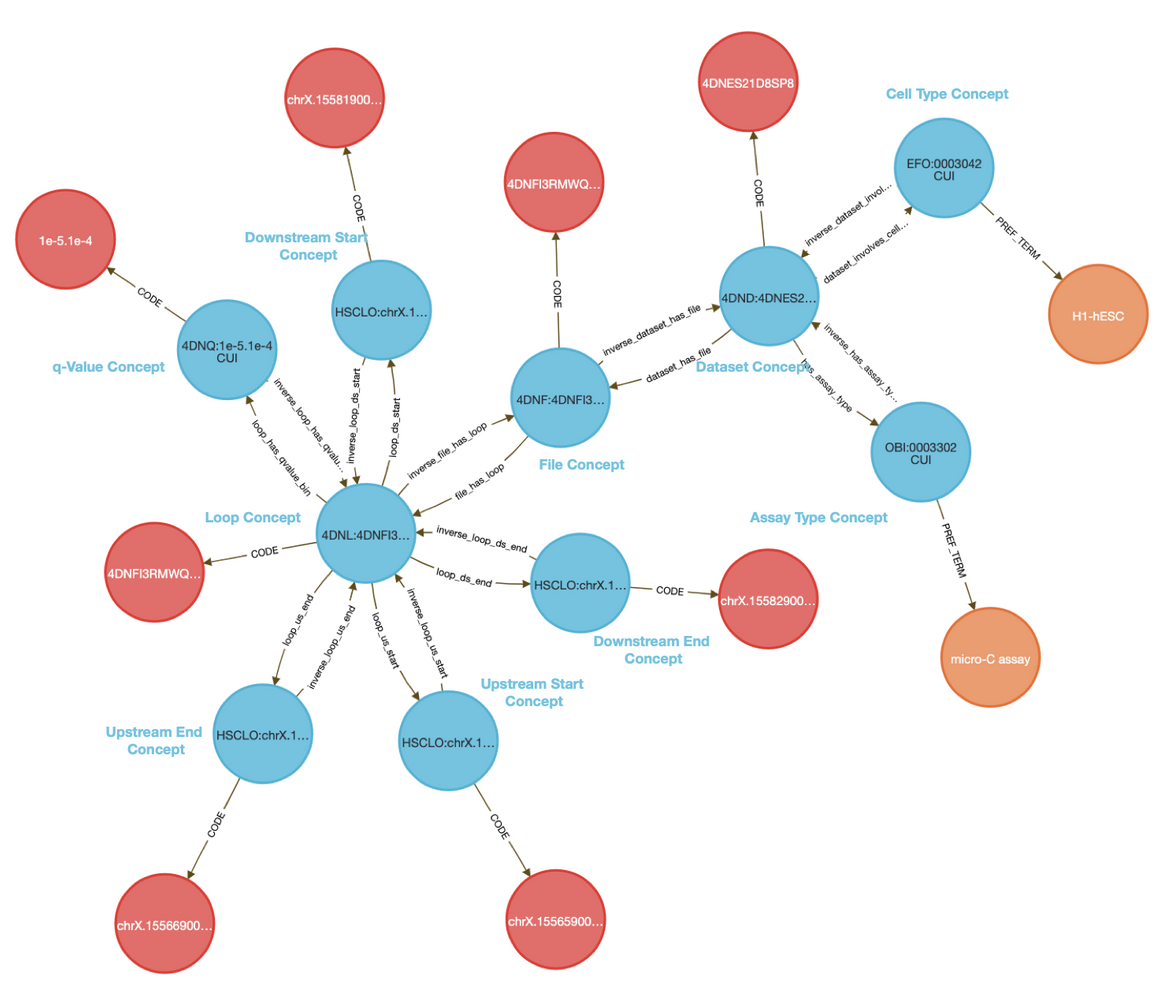
CFDE DD-Knowledge Graph
The CFDE Data Distillery Knowledge Graph contains entities and relationships across the CFDE. View Cyclic gmp's neighborhood in the knowledge graph.
GDLPA Landing Pages Links
The Gene and Drug Landing Page Aggregator (GDLPA) finds links to primary and secondary source information from CFDE and other resources. Discover landing pages for Cyclic gmp.
Playbook Workflow Builder
The Playbook Workflow Builder helps you interactively construct workflows leveraging CFDE APIs without code. Start a new workflow with Cyclic gmp.
Results found
Linked to
| Label | Description |
|
|---|---|---|---|
Metadatata for cyclic gmp | |||
A Knowledge Graph Assertion from IDG | |||
A Knowledge Graph Assertion from IDG |
Metadatata for cyclic gmp
A Knowledge Graph Assertion from IDG
A Knowledge Graph Assertion from IDG
DISPLAY PER PAGE
10
@CFDE Workbench 2026
The CFDE Workbench is actively being developed and maintained by the CFDE Data Resource Center (DRC).The DRC is funded by OT2OD036435 from the Common Fund at the National Institutes of Health.
The CFDE Workbench is actively being developed and maintained by the CFDE Data Resource Center (DRC).The DRC is funded by OT2OD036435 from the Common Fund at the National Institutes of Health.
@CFDE Workbench 2026