Irbesartan (Compound)
Synonyms:irbesartan, 138402-11-6, Avapro, Aprovel, Karvea, SR-47436, BMS-186295, Irbesartan BMS, SR 47436, BMS 186295, Irbesartan Zentiva, Irbesartan D4, Sarbevel, Irbesartan teva, Irbesartan-d3, Irbesartan free base, NSC-758696, CHEMBL1513, 2-butyl-3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]-1,3-diazaspiro[4.4]non-1-en-4-one, 2-butyl-3-{[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1,3-diazaspiro[4.4]non-1-en-4-one, 8-butyl-7-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]-7,9-diazaspiro[4.4]non-8-en-6-one, CHEBI:5959, DTXSID0023169, J0E2756Z7N, 138402-11-6 (free base), 2-Butyl-3-(p-(o-1H-tetrazol-5-ylphenyl)benzyl)-1,3-diazaspiro(4.4)non-1-en-4-one, 1,3-Diazaspiro(4.4)non-1-en-4-one, 2-butyl-3-((2'-(1H-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl)-, NCGC00095122-01, 1,3-Diazaspiro[4.4]non-1-en-4-one, 2-butyl-3-[[2'-(2H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-, DTXCID903169, Irbetan, Irbesartan Krka, [3H]irbesartan, 3-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-1,3-diazaspiro[4.4]non-1-en-4-one, Irbesartan Winthrop, SMR000466306, Avapro (TN), CAS-138402-11-6, 1185120-76-6, 2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl}-1,3-diazaspiro[4.4]non-1-en-4-one, UNII-J0E2756Z7N, 3-((2'-(1H-Tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-2-butyl-1,3-diazaspiro[4.4]non-1-en-4-one, HSDB 8215, Irbesartan [USAN:USP:INN:BAN], Irbesartan ,(S), 2-butyl-3-[[4-[2-(1H-tetrazol-5-yl)phenyl]phenyl]methyl]-1,3-diazaspiro[4.4]non-1-en-4-one, 3-((2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-1,3-diazaspiro[4.4]non-1-en-4-one, Irbesartan- Bio-X, Irbesartan (Avapro), MFCD00864464, Spectrum_001751, IRBESARTAN [MI], IRBESARTAN [INN], IRBESARTAN [JAN], Spectrum2_001675, Spectrum3_000994, Spectrum4_001122, Spectrum5_001288, IRBESARTAN [USAN], IRBESARTAN [VANDF], IRBESARTAN [MART.], SCHEMBL4246, IRBESARTAN [USP-RS], IRBESARTAN [WHO-DD], BSPBio_002687, GTPL589, KBioGR_001603, KBioSS_002231, MLS000759408, MLS001424099, BIDD:GT0347, IRBESARTAN [EMA EPAR], SPECTRUM1504259, SPBio_001889, GTPL6908, Irbesartan (JP17/USP/INN), IRBESARTAN [EP IMPURITY], IRBESARTAN [ORANGE BOOK], KBio2_002231, KBio2_004799, KBio2_007367, KBio3_001907, IRBESARTAN [EP MONOGRAPH], IRBESARTAN [USP IMPURITY], BCPP000202, HMS1922J05, HMS2051L08, HMS2093E16, HMS2232F23, HMS3370B06, HMS3393L08, HMS3715L04, IRBESARTAN [USP MONOGRAPH], Pharmakon1600-01504259, AVALIDE COMPONENT IRBESARTAN, BCP02004, HY-B0202, Tox21_111433, AC-537, BDBM50042235, CCG-39091, COAPROVEL COMPONENT IRBESARTAN, NSC758696, STK645362, AKOS005576396, AKOS015895353, IFIRMACOMBI COMPONENT IRBESARTAN, IRBESARTAN COMPONENT OF AVALIDE, Irbesartan, >=98% (HPLC), powder, Tox21_111433_1, AB07472, AM90289, BCP9000792, CCG-101012, DB01029, KS-1151, NC00262, NSC 758696, 2-Butyl-3-[2'-(1H-tetrazol-5-yl)biphenyl-4-ylmethyl]1,3-diaza-spiro[4.4]non-1-en-4-one, IRBESARTAN COMPONENT OF COAPROVEL, IRBESARTAN COMPONENT OF KARVEZIDE, NCGC00095122-02, NCGC00095122-03, NCGC00095122-04, NCGC00095122-05, NCGC00095122-14, 2-BUTYL-3-[[2'-(2H-TETRAZOL-5-YL)[1,1'-BIPHENYL]-4-YL]METHYL]-1,3-DIAZASPIRO[4.4]NON-1-EN-4-ONE, BI164582, IRBESARTAN COMPONENT OF IFIRMACOMBI, SBI-0206769.P001, FT-0601598, FT-0670413, I0859, S1507, SR-47436;BMS-186295, C07469, D00523, AB00639954-06, AB00639954_07, AB00639954_08, A807387, EN300-37158602, L000319, Q947266, SR-05000001997, Q-201249, SR-05000001997-1, BRD-K60038276-001-02-5, BRD-K60038276-001-03-3, IBERSARTAN/HYDROCHLOROTHIAZIDE COMPONENT IBERSARTAN, Irbesartan, European Pharmacopoeia (EP) Reference Standard, IBERSARTAN/HYDROCHLOROTHIAZIDE TEVA COMPONENT IBERSARTAN, Irbesartan, United States Pharmacopeia (USP) Reference Standard, IBERSARTAN COMPONENT OF IBERSARTAN/HYDROCHLOROTHIAZIDE TEVA, IBERSARTAN COMPONENT OF IBERSARTAN/HYDROCHLOROTHIAZIDE ZENTIVA, IBERSARTAN/HYDROCHLOROTHIAZIDE ZENTIVA COMPONENT IBERSARTAN, Irbesartan, Pharmaceutical Secondary Standard; Certified Reference Material, 2-(n-butyl)-3-[[2'-(tetrazol-5-yl)biphenyl-4-yl]methyl]-1,3-diazaspiro[4.4]non-1-en-4-one, 2-butyl-3-({4-[2-(2H-1,2,3,4-tetrazol-5-yl)phenyl]phenyl}methyl)-1,3-diazaspiro[4.4]non-1-en-4-one, 2-Butyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-1,3-diaza-spiro[4.4]non-1-en-4-one, 2-butyl-3-{[2''-(1H-tetrazol-5-yl)[1,1''-biphenyl]-4-yl]methyl}-1,3-diazaspiro[4.4]non-1-en-4-one, 2-butyl-3-{[2''-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1,3-diazaspiro[4.4]non-1-en-4-one, 2-butyl-3-{[2'-(2H-1,2,3,4-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl]methyl}-1,3-diazaspiro[4.4]non-1-en-4-one, 3-((2'-(1H-Tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)-methyl)-2-butyl-1,3-diazaspiro[4.4]non-1-en-4-one, 8-butyl-7-[[4-[2-(2H-1,2,3,4-tetrazol-5-yl)phenyl]phenyl]methyl]-7,9-diazaspiro[4.4]non-8-en-6-one
Id:3749
Description:nan
Analyze
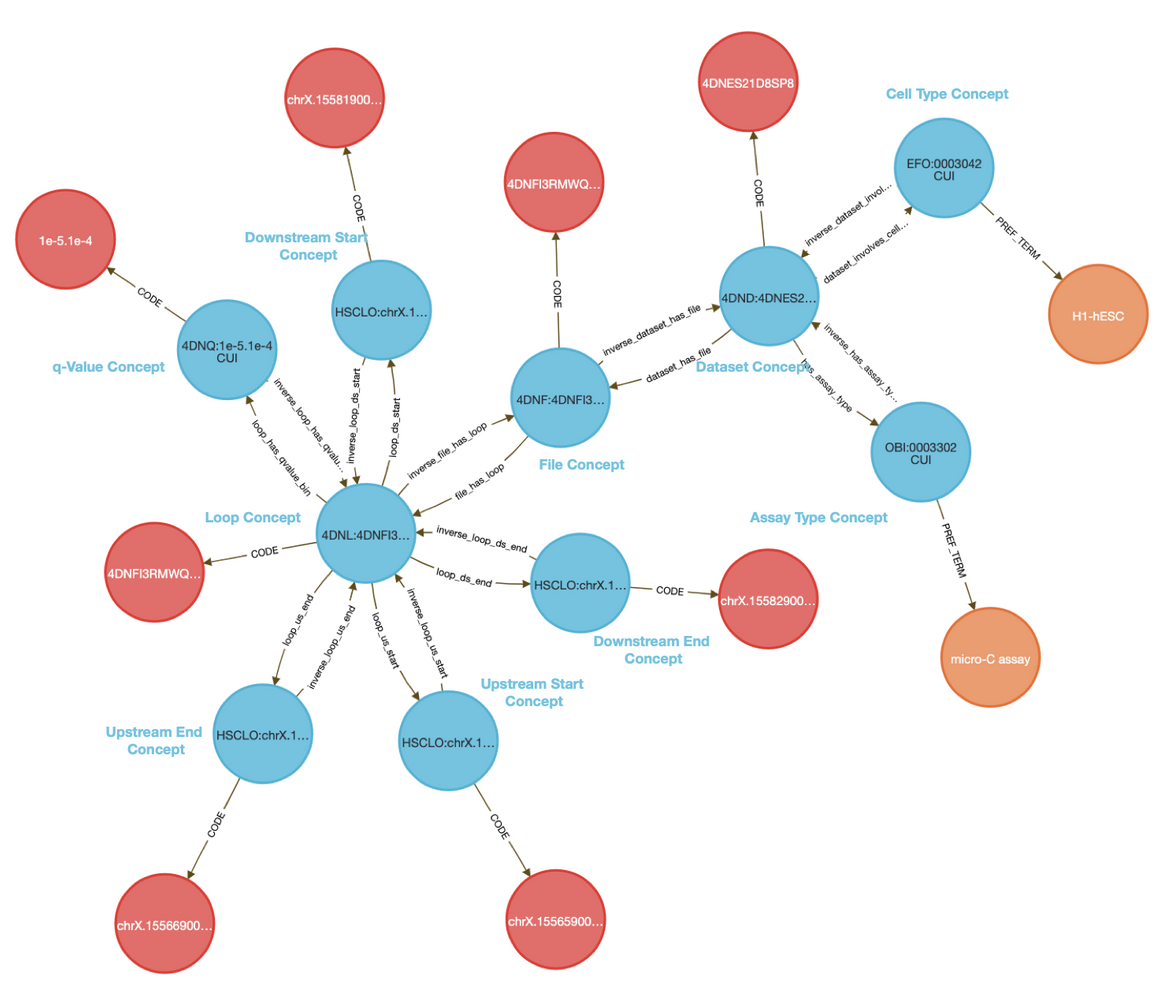
CFDE DD-Knowledge Graph
The CFDE Data Distillery Knowledge Graph contains entities and relationships across the CFDE. View Irbesartan's neighborhood in the knowledge graph.
GDLPA Landing Pages Links
The Gene and Drug Landing Page Aggregator (GDLPA) finds links to primary and secondary source information from CFDE and other resources. Discover landing pages for Irbesartan.
Playbook Workflow Builder
The Playbook Workflow Builder helps you interactively construct workflows leveraging CFDE APIs without code. Start a new workflow with Irbesartan.
Results found
Linked to
| Label | Description |
|
|---|---|---|---|
A Substance | |||
Metadatata for irbesartan | |||
DrugPage Collection: (DrugCentralID:01481; PubChem_CID:3749, FILE=drugcentral_drug_01481.json) |
Metadatata for irbesartan
DrugPage Collection: (DrugCentralID:01481; PubChem_CID:3749, FILE=drugcentral_drug_01481.json)
DISPLAY PER PAGE
10
@CFDE Workbench 2026
The CFDE Workbench is actively being developed and maintained by the CFDE Data Resource Center (DRC).The DRC is funded by OT2OD036435 from the Common Fund at the National Institutes of Health.
The CFDE Workbench is actively being developed and maintained by the CFDE Data Resource Center (DRC).The DRC is funded by OT2OD036435 from the Common Fund at the National Institutes of Health.
@CFDE Workbench 2026